Temperature
Temperature is a physical quantity that expresses hot and cold or a measure of the average kinetic energy of the atoms or molecules in the system. It is the manifestation of thermal energy, present in all matter, which is the source of the occurrence of heat, a flow of energy, when a body is in contact with another that is colder or hotter. Temperature should not be confused with heat.
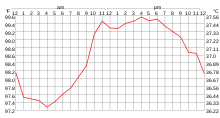
Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have used various reference points and thermometric substances for definition. The most common scales are the Celsius scale (formerly called centigrade, denoted as °C), the Fahrenheit scale (denoted as °F), and the Kelvin scale (denoted as K), the last of which is predominantly used for scientific purposes by conventions of the International System of Units (SI).
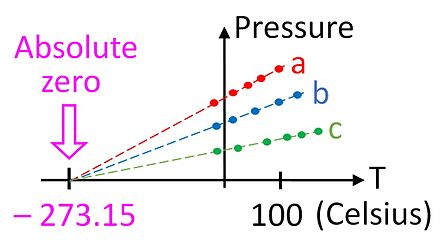
The lowest theoretical temperature is absolute zero, at which no more thermal energy can be extracted from a body. Experimentally, it can only be approached very closely (100 pK), but not reached, which is recognized in the third law of thermodynamics.
Temperature is important in all fields of natural science, including physics, chemistry, Earth science, astronomy, medicine, biology, ecology, material science, metallurgy, mechanical engineering and geography as well as most aspects of daily life.
Temperature scales differ in two ways: the point chosen as zero degrees and the magnitudes of incremental units or degrees on the scale.
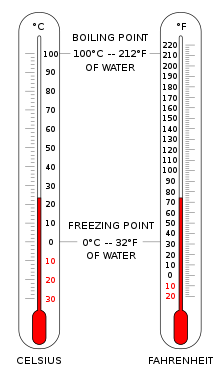
The Celsius scale (°C) is used for common temperature measurements in most of the world. It is an empirical scale that was developed by historical progress, which led to its zero point 0 °C being defined by the freezing point of water, and additional degrees defined so that 100 °C was the boiling point of water, both at sea-level atmospheric pressure. Because of the 100-degree interval, it was called a centigrade scale.[3] Since the standardization of the kelvin in the International System of Units, it has subsequently been redefined in terms of the equivalent fixing points on the Kelvin scale, and so that a temperature increment of one degree Celsius is the same as an increment of one kelvin, though they differ by an additive offset of exactly 273.15.